Principle of galvanic corrosion
Galvanic corrosion occurs as a result of many factors such as the contact of two metals with different potentials, ambient humidity, temperature, etc.
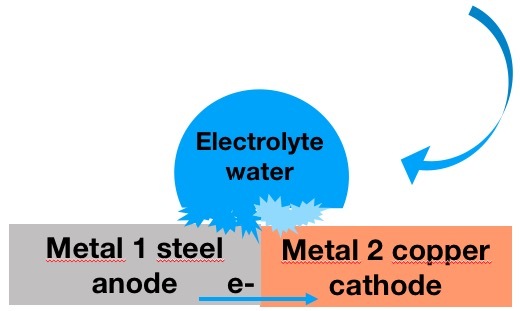
This electrochemical principle is similar to a short-circuited battery. The term galvanic designates an electric current generated by two different metals with displacement of metal ions. By electric current we mean a displacement of electrons (e-) from one metal to another.
This loss of electron (from the anode to the cathode) causes a structural modification of the metals.
Macroscopically this modification results in “during a contact of 2 different metals, the metal of the couple with the weakest electrochemical potential will be eroded”. The difference in electrochemical potential must therefore not be too high in order to ensure the structural durability of the bimetal installation.
Copper | Brass | Coppered steel | 304 SST Steel | 316 SST Steel | Steel | Galvanized Steel | Aluminium | Zinc |
|
|---|---|---|---|---|---|---|---|---|---|
| Copper | |||||||||
| Brass | |||||||||
| Coppered Steel | |||||||||
| 304 SST Steel | |||||||||
| 316 SST Steel | |||||||||
| Steel | |||||||||
| Galvanized steel | |||||||||
| Aluminium | |||||||||
| Zinc |
Very low galvanic torque, safe to use.
Low galvanic torque.
High galvanic torque, not recommended or protection recommended.
Example:
Cu2 + / Cu (Cu the metal “copper” and Cu2 + the ion “copper II”)
Electrochemical potential = 0.34 Volt
Zn2 + / Zn (Zn the metal Zinc and Zn2 + the ion “Zinc II”)
Electrochemical potential = -0.76 Volt
Here we will have Zinc which will be oxidized to Zn2 + (ie it will be “eaten away”).
This example of simple corrosion occurs in the presence of water.
In industry, the principle of dry corrosion is more often observed. This corrosion is similar but the oxides deposited are solid. Example with Iron and its rust (iron oxide) Fe2O3.
To avoid this corrosion, it is necessary to choose metal couples whose galvanic couples are the closest and to avoid an unfavorable surface ratio (surface of the cathode always smaller than the anode).
If any question, feel free to contact us, we will be pleased to give you further information.

